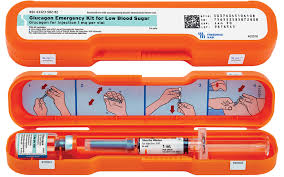
FDA approved the first generic glucagon injection kit (manufactured by Amaphastar Pharmaceuticals) which is therapeutically equivalent to Eli Lilly’s branded Glucagon injection kit. The generic injection kit is approved to treatment severe hypoglycemia (typically defined as blood glucose < 54 mg/dL) and as a diagnostic test (in gastrointestinal (aka stomach related) radiologic imaging). It is similar to Lilly’s branded glucagon kit in that it involves a multistep mixing process. The advantage the generic has over the branded injection kit is a lower cost although the mixing is still cumbersome (when considering its use for severe hypoglycemia). The newer products (which are not available generically) have the advantage of ease of use with either the intranasal (Baqsimi) or prefilled pen or syringe (Gvoke HypoPen and Gvoke PFS) which personally I would prefer in an emergency of severe hypoglycemia. Please click below to learn more.

Please share your thoughts and subscribe to receive my blogs.
#generic #glucagon #FDA #approval
Follow me on Twitter and Facebook @ReecesPiecesDi and Instagram.
