Contributor: Zuhal Saadut, PharmD Candidate 2025, PCOM Georgia School of Pharmacy
Meet Jana, a 48-year-old female that was recently diagnosed with prediabetes at her most recent primary care visit, with an A1c of 6.3%. She was upset when she found out about her diagnosis as her father had type 2 Diabetes. He had several incidences of extreme hyperglycemia, or high blood sugar, that required hospitalization. Her father also experienced vision loss due to many years of poor blood sugar control. Jana is afraid of these things potentially happening to her and wants to keep a close eye on her blood sugar levels without having to prick her finger every day and carry a blood glucose meter kit everywhere she goes. She is determined to gain control of her health again and signed up for nutrition classes and a gym membership to improve her dietary intake and physical activity. With these lifestyle changes, she wants to see how fast it will work. Jana is curious about the use of continuous glucose monitoring but does not want to go through the prescription process because her “insurance sucks anyways”.
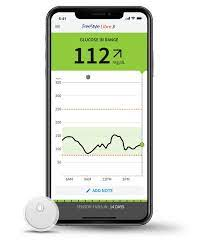
As of March 5th, 2024, the U.S. Food and Drug Administration (FDA) has approved its first over the counter (OTC) continuous glucose monitoring (CGM), the Dexcom Stelo Glucose Biosensor. This product is intended for individuals ages 18 and older that either do not use insulin to treat their diabetes or want to check their blood glucose trends as they eat or exercise. This system does not have the ability to warn patients if they are experiencing hypoglycemia, or low blood sugar (<70 mg/dL), therefore is not meant for individuals with problematic hypoglycemia.
Dexcom Stelo is a sensor that is applied in a similar fashion as other CGM sensors, with wear time that can last up to 15 days. A study was conducted on the sensor wear time and showed that 77.9% of sensors last the full 15 days. The remaining sensors did not last for 15 days, with 10% of them lasting less than 12 days. The sensor must be paired with a smartphone device that is compatible with the Dexcom application to be downloaded. A Dexcom account must also be created before the sensor can be used. The readings of your blood sugar will appear on the app and updates every 15 minutes. Dexcom Stelo is currently not available in the market, but according to their manufacturers, it should be available Summer 2024 for online purchase.
Abbott has also obtained FDA approval for OTC CGMs (Lingo and Libre Rio) as of June 10th, 2024. Lingo is intended for individuals ages 18 and older that are looking to improve their overall health and wellness, with a sensor wear time of 14 days. Libre Rio is intended for individuals ages 18 and older with Type 2 Diabetes that do not use insulin therapy, with sensor wear time of up to 15 days, measuring blood sugars ranging from 40 to 400 mg/dL. Both of these sensors require smartphone devices compatible with their respective applications and an account must be created before use of the sensor. Neither product from Abbott is available on the market yet, however, Lingo is estimated to be available for purchase Summer 2024. As of right now, there is no estimated time of when Libre Rio will be available for purchase.
The OTC CGMs differ from the prescription CGM as they have an age restriction and are recommended to not be used in individuals with type 1 diabetes or those with type 2 Diabetes on insulin therapy or incidences of hypoglycemia. If you notice constant high or low readings of your blood sugar, be sure to consult your healthcare provider before taking any action of adjusting your medication doses. Adverse effects of sensor use include local infection, skin irritation and pain or discomfort.
Fast forward to 6 months later, she was looking forward to her follow up appointment with her doctor to find out what her new A1c would be after these lifestyle modifications. Results came back with an A1c of 5.3%! Her doctor applauded her for taking the initiative for her health. Jana is relieved that she was able to prevent herself from getting Diabetes before it was too late and will continue to do her best to stay healthy. Jana is happy that she has the option to monitor her blood sugar levels without having to prick her finger daily and loves that the results are digital on her phone.
References:
- https://www.fda.gov/news-events/press-announcements/fda-clears-first-over-counter-continuous-glucose-monitor
- https://www.dexcom.com/stelo?sfc=7014y000000zbBPAAY&gad_source=1&gclid=CjwKCAjwm_SzBhAsEiwAXE2Cv2_hWxtpvxHKBeCsnM7QgiPMyrEyYy6GPT3gTJun9hBN2PF96-UH7RoCBmAQAvD_BwE