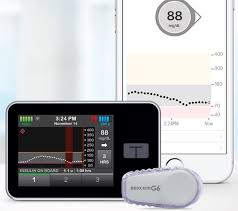
FDA’s approval of Tandem’s Control-IQ, automated insulin delivery (AID) system, is game changer. Why? It is the first insulin dosing software to win fast approval by FDA “interoperability” approval pathway. The interoperable automated glycemic controller device automatically adjusts insulin delivery by connecting to an alternate controlled enabled pump (ACE pump) and integrated continuous glucose monitor (iCGM). Automated insulin dosing (AID) system typically consists of pump, CGM and software to control system of compatible devices. With regards to Tandem, Control-IQ combines Tandem’s t:slim X2 insulin pump, Dexcom G6 CGM and algorithm built into the pump to give automatic bolus doses and adjust basal insulin delivery.
New Tandem pumps with advanced hybrid closed loop algorithm Control-IQ will ship by end of January 2020.
https://www.tandemdiabetes.com/products/t-slim-x2-insulin-pump/control-iq

Please share your thoughts and subscribe to receive my blogs.
#FDA #Control-IQ #Interoperability #Tandem
Follow me on Twitter and Facebook @ReecesPiecesDi and Instagram.
