Contributor: Airam Viennelu Aliwalas, PharmD Candidate 2027, PCOM Georgia School of Pharmacy
Helen, a 50-year-old woman, with a 3-year history of type 2 diabetes mellitus, presents to the clinic for her 3-month follow-up on therapy management. She is currently on insulin glargine (Lantus), a long-acting insulin, for the management of her type 2 diabetes mellitus. During her visit, she stated concern about the cost of her medications and inquired about alternatives that are as effective but more affordable than her current regimen.
Insulin is a hormone released by the pancreas to regulate the body’s blood sugar levels by allowing glucose to enter cells to be used as energy. Per the 2025 American Diabetes Association (ADA) Standards of Care in Diabetes, synthetic insulin therapy is required for type 1 diabetes mellitus, where the body does not produce enough insulin, and for specific parameters in type 2 diabetes mellitus, where the body does not use insulin effectively.
According to the National Institutes of Health (NIH), a biosimilar refers to a product that is clinically similar to an already approved reference biologic drug in terms of efficacy and safety. These products typically cost less than the reference drug, allowing the medication to be more affordable and accessible, especially for chronic conditions like diabetes mellitus. The approval process in the United States surrounding biosimilars requires preclinical and clinical studies to show that the proposed product to be equally effective as the reference product and are regulated by the Food and Drug Administration (FDA).
Approved Biosimilar and Follow-on Biologics in the United States
| Reference Insulin | Biosimilar/Follow-on Insulin | Manufacturer | FDA Approval |
| Insulin Glargine | Insulin glargine (Basaglar) | Eli Lilly | 2015 as follow-on insulin |
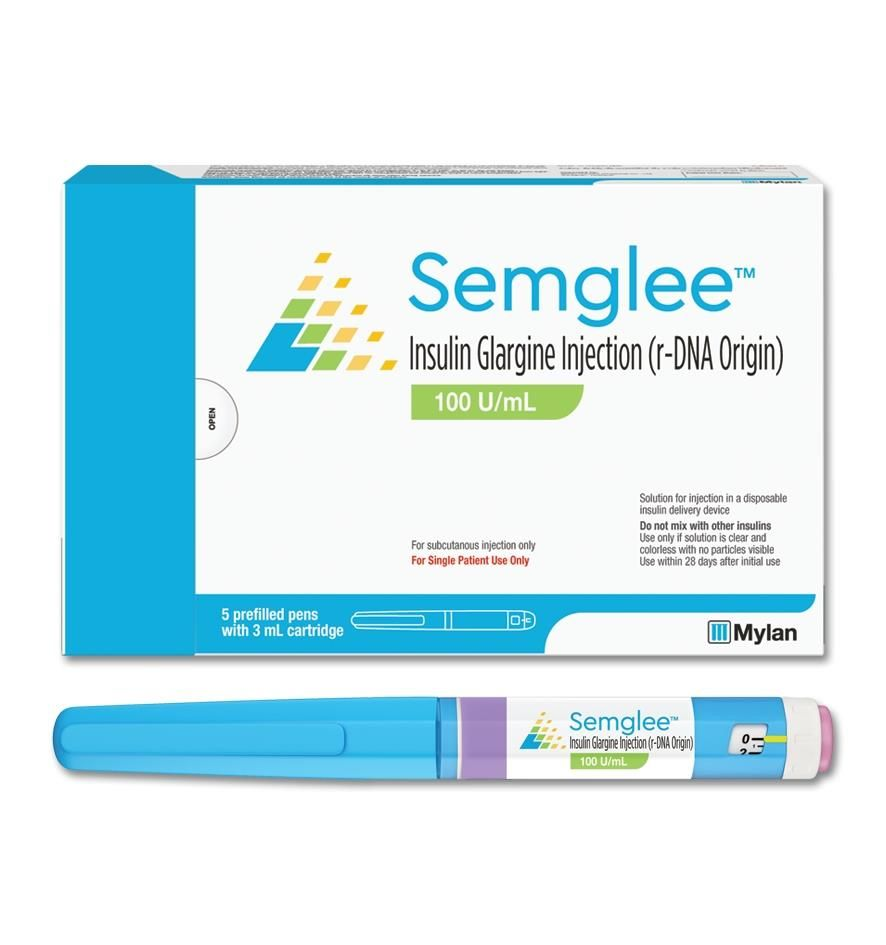
| Insulin Glargine – yfgn (Semglee) | Viatris | 2020 as follow-on insulin 2021 as biosimilar | |
| Insulin Lispro | Insulin Lispro (Admelog) | Sanofi Aventis | 2017 as follow-on insulin |
| Insulin Aspart | Insulin Aspart-szjj (Merilog) | Sanofi Aventis | 2025 as biosimilar |
With Helen’s concern regarding the price of her medication, insulin Glargine (Lantus), her healthcare team discussed possible alternatives while considering her insurance coverage. Her provider offered to switch her medication to the biosimilar, Semglee, to which Helen agreed. At her 3-month follow-up visit, her A1c remained stable and she expressed relief from financial stress.
References
Heinemann L, Davies M, Home P, Forst T, Vilsbøll T, Schnell O. Understanding biosimilar insulins – development, manufacturing, and clinical trials. Journal of diabetes science and technology. November 2023. Accessed April 18, 2025.
American Diabetes Association. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2025 . Accessed April 18, 2025.

