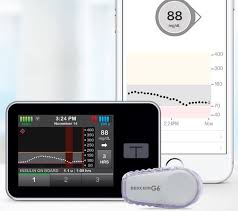
Technology in the form in insulin pumps, CGMs and apps have transformed the daily living with diabetes. Insulin pumps and CGMs are invaluable tools for pediatric and adult persons living with diabetes. Having options in terms of the best insulin pump and CGM for the individual living with diabetes is key. Now pediatrics specifically children 6 – 13 years old have the option of Tandem Control – IQ, hybrid closed loop system, as the FDA has expanded approval to this age group. Please click below to learn more about this exciting expanded approval of Tandem Control – IQ.
https://diatribe.org/tandems-control-iq-cleared-ages-6-13-automated-insulin-delivery-children
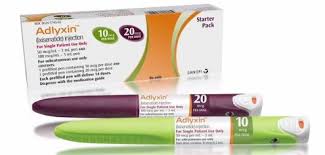
Recently published data from trial with lixisenatide, short acting (daily injection) GLP-1 agonist, showed a significant delay in gastric emptying and reduction of postprandial glucose. Lixisenatide has a role in significantly reducing postprandial glucose. Please click below to learn more about the trial data.

Please share your thoughts and subscribe to receive my blogs.
#FDA #Pediatrics #Tandem #ControlIQ #Lixisenatide
Follow me on Twitter and Facebook @ReecesPiecesDi and Instagram.
