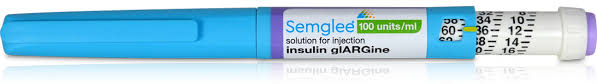
FDA has approved insulin glargine (Semglee, U-100) manufactured by Mylan Pharmaceuticals this week. We now have 3 insulin glargine U-100 (Lantus, Basaglar and Semglee) formulations available. Semglee is approved for use in adults and pediatrics with type 1 diabetes and adults with type 2 diabetes.
FDA announces additional companies to being asked to voluntarily recall metformin extended release due to elevated levels of NDMA. Additional details in link below.

Please share your thoughts and subscribe to receive my blogs.
#FDA #Semglee #metformin #NDMA
Follow me on Twitter and Facebook @ReecesPiecesDi and Instagram.
