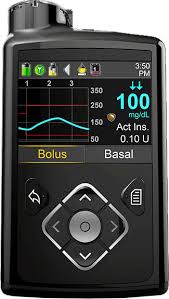
Unfortunately, we have two recalls this week. FDA has issues a class I recall (most serious) for certain Medtronic MiniMed 600 series (please click on link below for specifics) due to incorrect insulin dosing which has caused 2,175 injuries and one death.
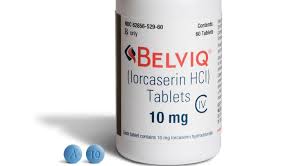
Secondly, Eisai manufacturer of weight loss medication, lorcaserin (Belviq), has agreed to voluntarily withdrawal lorcaserin from the market due to concerns of cancer risk.

Please share your thoughts and subscribe to receive my blogs.
#FDA #recall #Medtronic #Belviq
Follow me on Twitter and Facebook @ReecesPiecesDi and Instagram.
